The immune
system is a system of
biological structures and processes within an organism that protects against disease. To function properly, an immune system must detect a wide
variety of agents, from viruses to parasitic worms, and distinguish them from the organism's own healthy tissue.
HEMATOPOIESIS
Hematopoiesis is the formation of blood cellular
components. All cellular blood components are derived from hematopoietic stem
cells. In a healthy adult person, approximately 1011–1012 new blood cells are produced daily in
order to maintain steady state levels in the peripheral circulation.
Hematopoietic
stem cells (HSCs) reside in the medulla of the bone (bone marrow)
and have the unique ability to give rise to all of the different mature blood
cell types and tissues. HSCs are self-renewing cells: when they proliferate, at
least some of their daughter cells remain as HSCs, so the pool of stem cells
does not become depleted. The other daughters of HSCs (myeloid and lymphoid
progenitor cells), however can each commit to any of the alternative
differentiation pathways that lead to the production of one or more specific
types of blood cells, but cannot self-renew. This is one of the vital processes
in the body.
CELLS OF THE IMMUNE SYSTEM
All cells of the immune system originate from a
hematopoietic stem cell in the bone marrow, which gives rise to two major
lineages, a myeloid progenitor cell and a lymphoid progenitor cell (Figure
4). These two progenitors give rise to the myeloid cells (monocytes,
macrophages, dendritic cells, meagakaryocytes and granulocytes) and lymphoid
cells (T cells, B cells and natural killer (NK) cells), respectively.
Theses cells make up the cellular components of the innate (non-specific) and
adaptive (specific) immune systems.
Cells of
the innate immune system
Cells of the innate immune system include
phagocytic cells (monocyte/macrophages and PMNs), NK cells, basophils, mast
cells, eosinophiles and platelets. The roles of these cells have been
discussed previously the receptors of these cells are pattern recognition
receptors (PRRs) that recognize broad molecular patterns found on pathogens
(pathogen associated molecular patterns, PAMPS).
Cells
that link the innate and adaptive immune systems
A specialized subset of cells
called antigen presenting cells (APCs) are a heterogeneous population of
leukocytes that play an important role in innate immunity and also act as a
link to the adaptive immune system by participating in the activation of helper
T cells (Th cells). These cells include dendritic cells and
macrophages. A characteristic feature of APCs is the expression of a cell
surface molecule encoded by genes in the major histocompatibility complex,
referred to as class II MHC molecules. B lymphocytes also express
class II MHC molecules and they also function as APCs, although they are not
considered as part of the innate immune system. In addition, certain
other cells (e.g., thymic epithelial cells) can express class II
MHC molecules and can function as APCs.
Cells of the adaptive immune system
Cells that make up the
adaptive (specific) immune system include the B and T lymphocytes. After
exposure to antigen, B cells differentiate into plasma cells whose primary
function is the production of antibodies. Similarly, T cells can
differentiate into either T cytotoxic (Tc) or T helper (Th) cells of which
there are two types Th1 and Th2 cells.
There are a number of
cell surface markers that are used in clinical laboratories to distinguish B
cells, T cells and their subpopulations.
MONOCYTES
Monocytes are a type of white blood cell and are
part of the innate immune system of vertebrates including all mammals (humans
included), birds, reptiles, and fish. Monocytes play multiple roles in immune
function. Monocytes are usually identified in stained smears by their large
kidney shaped or notched nucleus. Monocytes are produced by the bone marrow
from hematopoietic stem cell precursors called monoblasts. Monocytes circulate
in the bloodstream for about one to three days and then typically move into
tissues throughout the body. They constitute between three to eight percent of
the leukocytes in the blood. Half of them are stored as a reserve in the
spleen. In the tissues, monocytes mature into different types of macrophages at
different anatomical locations. Monocytes are the largest corpuscles in the
blood.
Monocytes
which migrate from the bloodstream to other tissues will then differentiate
into tissue resident macrophages.
MACROPHAGES
Macrophages are cells produced by the
differentiation of monocytes in tissues. Macrophages were discovered by Ilya
Mechnikov, a Russian bacteriologist, in 1884. Human macrophages are about 21 micrometers
(0.00083 in) in diameter. Monocytes and macrophages are phagocytes. Macrophages
function in both non-specific defense (innate immunity) as well as help
initiate specific defense mechanisms (adaptive immunity) of vertebrate animals.
Their role is to phagocytose, or engulf and then digest, cellular debris and
pathogens, either as stationary or as mobile cells. They also stimulate
lymphocytes and other immune cells to respond to pathogens. They are
specialized phagocytic cells that attack foreign substances, infectious
microbes and cancer cells through destruction and ingestion. They move by
action of amoeboid movement. Macrophages are highly specialized in removal of
dying or dead cells and cellular debris. They are normally found in the liver,
spleen, and connective tissues of the body.
Killing mechanism (Phagocytosis)
Sequence
of Events:
Phagocytosis begins with the
neutrophil or macrophage flowing around the pathogen and engulfing it so that
it winds up enclosed in a phagosome (phagocytic vesicle). But this is
only the first step, because the more challenging task of destroying the
microorganisms remains. Indeed, some pathogens have special, effective
mechanisms for frustrating this destruction step.
The next step is the fusion of lysosomes with the
phagosome. The result is called a phagolysosome. Lysosome are derived
from the Golgi apparatus, much like secretion vesicles, but their contents are
focused on destroying microorganisms.
Destruction
of the Microbes: The
following are important factors that help destroy microorganisms within a
phagolysosome:
- Oxygen Radicals.
A complex of proteins called phagocyte oxidase in
the membrane of a phagolysosome generates oxygen radicals in the
phagosome. A single electron is taken from NADPH and added to oxygen,
partially reducing it. The resulting highly reactive molecules react with
proteins, lipids and other biological molecules. See the next webpage for
details.
- Nitric Oxide.
Nitric oxide synthase synthesizes nitric oxide, a
reactive substance that reacts with superoxide to create further molecules
that damage various biological molecules. (But nitric oxide is also,
remarkably enough, an important regulatory molecule elsewhere. More on
this later this quarter.)
- Anti-Microbial Proteins. Lysosomes contain several proteases,
including a broad spectrum enzyme, elastase, which is important or
even essential for killing various bacteria. Another anti-microbial
protein is lysozyme, which attacks the cell walls of certain (gram
positive) bacteria.
- Anti-Microbial Peptides. Defensins
and certain other peptides attack bacterial cell membranes. Similar
molecules are found throughout much of the animal kingdom.
- Binding Proteins.
Lactoferrin binds
iron ions, which are necessary for growth of bacteria. Another protein
binds vitamin B12.
- Hydrogen Ion Transport.
Transporters for hydrogen ions (a second role of the oxidase) acidify the
phagolysosome, which kills various microorganisms and is important for the
action of the proteases described above.
Release of Regulatory Molecules: In
addition to destroying the microorganism, phagocytes also release molecules
that diffuse to other cells and help coordinate the overall response to an infection.
Regulatory molecules that regulate an immune response are called cytokines.
Most are small proteins and are mainly released by white blood cells and their
relatives, such as macrophages.
Identification
of Pathogen: We will go into
this topic in more detail later. But here are a few points for now. Neutrophils
and macrophages have some ability on their own to recognize microorganisms and
begin phagocytosis. We will use the term innate receptors for the
molecules on such cells that available immediately to bind foreign molecules.
These can act as soon as a microbe enters the body. They are naturally found on
the surface of phagocytes and do not require a specific immune response to be
made. Innate receptors are possible because microorganisms have various
molecules on their surfaces that much different than those found in a human.
But phagocytosis is far more effective if microorganisms are labelled by
special molecules that bind to their surface. Any molecule that binds to a
microorganism and thereby speeds phagocytosis is called an opsonin. Most
important here are antibodies (such as IgG), which specifically identify
molecules at the surface of specific microorganisms. With this attached to the
surface of the microorganisms, phagocytosis is much more effective and rapid.
Difficult
Pathogens: But, as mentioned above, sometimes phagocytes
have a difficult time with certain pathogens. For example, Listeria
monocytogenes can escape from the phagosome into the cytosol. Tuberculosis
is an especially important example. A macrophage can usually engulf the
tuberculosis bacterium, but then the bacterium has a means for preventing the
lysosomes from fusing with the phagosome.
Dendritic cell
Dendritic cells
(DCs) are immune cells forming part of
the mammalian immune
system. Their main function is to process antigen material and present it
on the surface to other cells of the immune system. That is, dendritic cells
function as antigen-presenting cells. They act as
messengers between the innate and adaptive immunity.
Dendritic cells are present
in tissues in contact with the external environment, such as the skin (where there is
a specialized dendritic cell type called Langerhans cells) and the inner lining of the nose, lungs, stomach and intestines. They can also
be found in an immature state in the blood. Once activated,
they migrate to the lymph nodes where they interact with T cells and B cells to initiate and
shape the adaptive.
DISEASES:
HIV
infection: HIV,
which causes AIDS,
can bind to dendritic cells via various receptors expressed on the cell.
Autoimmunity Altered function of dendritic cells
is also known to play a major or even key role in allergy and autoimmune diseases.
NEUTROPHIL:
Neutrophils are the most abundant
white blood cells in humans. And are the first immune cells to react to
inflammation or infection via chemo taxis, internalizing and killing
microorganisms and ingesting particles through the process of phagocytosis.
Structure and function:
- Neutrophils, or polymorphnuclear neutrophils (PMN),
consist of 60-70% of the circulating leukocytes. They are the most
abundant type of blood cells in mammals.
- Immature neutrophils in circulation are non-segmented
and become segmented as they mature. They adhere to the endothelial cells
of the blood vessels and migrate through the endothelial cells via the
process of diapedesis.
- Neutrophils are highly active and play an important
role in acute inflammation. Their major roles are phagocytosis and
destruction of pathogens.
Cellular
components
Neutrophils consist of a 2-5 lobed nucleus joined together by hair like filaments. They have small golgi apparatus and mitochondria, with a small number of ribosomes and no rough endoplasmic reticulum. Neutrophils move in amoeboid motion by extending their pseudopodium that draws the nucleus and the rear of the cell forward
Neutrophils consist of a 2-5 lobed nucleus joined together by hair like filaments. They have small golgi apparatus and mitochondria, with a small number of ribosomes and no rough endoplasmic reticulum. Neutrophils move in amoeboid motion by extending their pseudopodium that draws the nucleus and the rear of the cell forward
. Neutrophils
have two types of stored antibiotic proteins:
·
The
primary (azurophilic) granules: these granules are lysosomal. They consist of
acidhydrolasesmyeloperoxidase and muramidase (lysozymes).
·
The
secondary (specific) granules: these granules are more specific to neutrophils
and they contain lactoferrin and lysozyme.
·
The
ingested microbe inside the vacuoles is called a phagosome and will fuse with
lysosomes to form phagolysosomes where the microbe will be destroyed.
EOSINOPHIL
The eosinophil is a specialized cell
of the immune system.Eosinophils become active when you have certain allergic
diseases, infections, and other medical conditions.
Structure
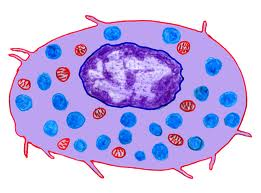
The eosinophil generally has a nucleus
with two lobes (bilobed), and cytoplasm filled with approximately 200 large
granules containing enzymes and proteins with different (known and unknown)
functions [see picture below; nucleus (purple), granules (pink)].
Location
Eosinophil are a normal cellular
component of the blood and also of certain tissues, including spleen, lymph
nodes, thymus, and the sub mucosal areas of the gastrointestinal, respiratory,
and genitourinary tracts. Counts of 0 to 450 eosinophil per cubic millimeter of
blood are generally considered within normal limits.
Development
Eosinophil are formed exclusively in
the bone marrow where they spend about 8 days in the process of maturation
before moving into the blood vessels. They travel through the vessels for 8 to
12 hours before they finally arrive at destination tissues, where they remain
for 1 to 2 weeks. Interleukin 5 (IL-5) is a major growth factor for this type
of cell.
Function
Eosinophil are pro-inflammatory white
blood cells that have many functions. They are implicated in numerous
inflammatory processes, especially allergic disorders. The functions of the
eosinophil are varied, some of which are very similar to other white cells.
Known functions include movement to inflamed areas, trapping substances,
killing cells, antiparasitic and bactericidal activity, participating in allergic
reactions, and modulating inflammatory responses.
Eosinophil granule proteins, such as
major basic protein (MBP), eosinophilic cationic protein (ECP), eosinophil
peroxidase (EPO) and eosinophil-derived neurotoxin (EDN), are capable of
inducing tissue damage and dysfunction. MBP, EPO and ECP have been shown to be
toxic to a variety of tissues including heart, brain, bronchial, and intestinal
epithelium. The degree of tissue injury is related to the duration of
eosinophilia, the level of eosinophil activation, and the type of stimulus
attracting the eosinophil.
There are many disorders where
eosinophils have been found elevated in the blood or in different tissues.
Given below are general categories of disease with examples of those that have
increased levels of eosinophils.
|
Allergic Disorders
|
Drug Reactions
|
Infectious Diseases
|
|
Blood Disorders
|
Immunologic Disorders and Reactions
|
Endocrine Disorders
|
|
Skin and Subcutaneous Disorders
|
Pulmonary Conditions
|
Gastrointestinal Diseases
|
|
Renal Diseases
|
Neurologic Disorders
|
Rheumatologic Illnesses
|
BASOPHIL
Basophil granulocytes, mostly referred
to as basophils, are the least common of the granulocytes, representing about
0.01% to 0.3% of circulating white blood cells. Basophils help protect the body
against disease and infections by eating some types of bacteria, foreign
substances, and other cells.
CHARACTERISTICS OF
BASOPHILS
1)
Basophils will look blA nucleus is the center of a cell. Thus, the
centers of basophils appear in various forms. A unique characteristic of
basophils, compared to other white blood cells, is that they usually do not
increase in numbers in response to sudden infections or diseases. However,
sometimes basophils will increase in response to sudden infections or
diseases.ack and blue or dark purple when stained by a basic dye, such as
Wright stain.
2)
Cytoplasm (a gel-like substance that fills up a cell) is mostly made of
many large, rough-looking, grain-like particles. These grain-like particles can
be seen under the microscope, but may sometimes cover the nucleus. The
grain-like particles may decrease in numbers in response to allergic reactions
and may increase in response to some types of inflammation.
3)
The grain-like particles of basophils contain the following substances:
histamine, leukotrienes, and heparin. Histamine is a substance found in all
cells that is released during allergic and inflammatory reactions. Leukotrienes
are substances in the body that produce allergic and inflammatory reactions,
similar to histamine. Heparin is a naturally occurring substance in the body
that prevents clotting (blood fluid coming together as a solid.
MAST CELL
Mast cells are cells found
throughout the body as part of our immune system. Mast cells appear to induce a
rapid inflammatory response to outside invaders, such as germs and viruses.
Mast cells play a large role in allergic responses, as they release the
chemical known as histamine.
The mast cell is very
similar in both appearance and function to the basophil, a type of white blood
cell. However, they are not the same, as they arise from different cell lines.
TYPES OF MAST CELL
Two types of mast cells
are recognized, those from connective tissue and a distinct set of mucosal mast
cells. The activities of the latter are dependent on T-cells.
CHARACTERISTICS OF MAST CELL
Mast cells store a number
of different chemical mediators including histamine, interleukins,
proteoglycans (e.g., heparin), and various enzymes—in coarse granules found
throughout the cytoplasm of the cell. Upon stimulation by an allergen.
The mast cells release the
contents of their granules (a process called degranulation) into the
surrounding tissues. The chemical mediators produce local responses
characteristic of an allergic reaction, such as increased permeability of blood
vessels (i.e., inflammation and swelling), contraction of smooth muscles (e.g.,
bronchial muscles), and increased mucus production.
Mast Cell Activation Disorder (MCAD)
What do chronic illnesses
such as Autism, Chronic Fatigue Syndrome (CFS), Fibromyalgia Lupus, Chronic
Lyme Disease, Interstitial Cystitis, Multiple Sclerosis, and more have in
common? Well, these illnesses may have a lot of things in common, and a lot of
overlapping symptoms, but many patients’ symptoms seem to be compatible with
Systemic Mast Cell Activation Disorder (MCAD).
LYMPHOCYTES
Lymphocytes are the central cells of the
immune system, responsible for adaptive immunity and the immunologic attributes
of diversity, specificity, memory, and self/non-self-recognition. The other
types of white blood cells play important roles, engulfing and destroying
microorganisms, presenting antigens, and secreting cytokines Lymphocytes
constitute 20%–40% of the body’s white blood cells and 99% of the cells in the
lymph. These lymphocytes continually circulate in the
blood and lymph and are capable of migrating into the tissue spaces and
lymphoid organs, thereby integrating the immune system to a high Degree The
lymphocytes can be broadly subdivided into three populations—B cells, T cells,
and null cells—on the basis of function and cell-membrane components.
NULL CELLS
They are may be of two types:
A. Killer cells
- Produce in bone
marrow.
- They do not
express the set of surface markers typical of B or T cells.
B. Natural killer cells (NK cells)
·
These are
large, granular lymphocytes.
·
It play a major role in defending the host
from both tumors and virally infected cells.
·
They were named "natural killer
cells" because of the initial motion that they do not require prior
activation in order to kill cells which are missing MHC class I.
·
NK cells distinguish infected cells and tumors
from normal and uninfected cells by recognizing changes of a surface molecule
called MHC (major histocompatibility complex) class I. NK cells are activated
in response to a family of cytokines called interferon.
T lymphocytes
·
Arise in the bone
marrow.
·
Unlike B cells, which mature within the bone
marrow, T cells migrate to the thymus gland to mature.
·
During its
maturation within the thymus, the T cell comes to express a unique
antigen-binding molecule, called the T-cell receptor, on its membrane
for antigen.
·
T-cell receptors can
recognize only antigen that is bound to cell-membrane proteins called major
histocompatibility complex (MHC) molecules.
·
MHC molecules that
function in this recognition event, which is termed “antigen presentation,” are
polymorphic (genetically diverse) glycoproteins found on cell membranes. They
play a central role in cell-mediated immunity.
·
They can be
distinguished from other lymphocytes, such as B cells and null cells, by the
presence of a T-cell receptor (TCR) on the cell surface.
There are well-defined subpopulations of T
cells:
A. T helper (TH) cells
·
Generally displaying
CD4 membrane molecule.
·
After a TH cell
recognizes and interacts with an antigen–MHC class II molecule complex, the
cell is activated—it becomes an effector cell that secretes various growth
factors known collectively as cytokines. The secreted cytokines play an
important role in activating B cells, TC cells, macrophages, and various other
cells that participate in the immune response.
·
Differences in the
pattern of cytokines produced by activated TH cells result in different types
of immune response.
B. T cytotoxic (TC) cells.
·
Displaying CD8
glycoprotein membrane molecule.
·
Under the influence
of TH-derived cytokines, a TC cell that recognizes an antigen–MHC class I
molecule complex proliferates and differentiates into an effector cell called a
cytotoxic T lymphocyte (CTL).
·
In contrast to the
TC cell, the CTL generally does not secrete many cytokines and instead exhibits
cell-killing or cytotoxic activity.
·
The CTL has a vital
function in monitoring the cells of the body and eliminating any that display
antigen, such as virus-infected cells, tumor cells, and cells of a foreign
tissue graft.
The ratio of TH to TC cells in a sample can
be approximated by assaying the number of CD4_ and CD8_ T cells. This ratio is
approximately 2:1 in normal human peripheral blood, but it may be significantly
altered by immunodeficiency diseases, autoimmune diseases, and other disorders.
In addition, most mature T cells express the
following membrane molecules:
- CD28, a receptor for the co-stimulatory B7 family of molecules present on
B cells and other antigen presenting cells
- CD45, a signal-transduction molecule.
C. T suppressor (TS) Cells
·
It maintain
tolerance to self-antigens cell.
·
T suppressor cell
are a component of the immune system that suppress immune responses of other
cells.
·
These cells
terminate the immune response when foreign bodies have been controlled or
destroyed.
D. T Memory cells
- These are a subset of antigen-specific T cells
that persist long-term after an infection has resolved.
- They quickly expand to large numbers of
effector T cells,
thus providing the immune system with "memory" against past
infections.
B lymphocytes
·
B cell
belong to white blood cell (WBCs) called lymphocytes.
WBCs protect the body from infection.
·
They are
called B cell because they mature in the bone marrow.
·
Main role is
to produce antibodies, also known as immunoglobulin, that circulates in the
blood & lymph.
·
And
activated when an infection occurs and produce antibodies that attach to the
surface of the infectious agent.
·
These
antibodies kill the infection causing organisms cell guard the immune system
against infection.
A. Plasma
cells:
·
Plasma cell is a type of white blood cell that
originates in the bone marrow.
·
These cells contribute antibodies to the immune
system and help protect the body against disease.
·
Plasma cells have a short lifespan, but generate
memory cells that live longer and can quickly fight recurrences of
infection.
·
The human body would not survive long without
their ability to generate antibodies.
B. The Memory Cells:
·
Memory
cell are the second cell type produced by the division of B cells.
·
These cells have a prolonged life span and can thereby
"remember" specific intruders. T cells can also produce memory cells
with an even longer life span than B memory cells.
·
The second time an intruder tries to invade the body, B and T
memory cells help the immune system to activate much faster.
·
The invaders are wiped out before the infected human feels any
symptoms. The
body has achieved immunity against the invader.
Conclusion:
The immune system is one of nature's more fascinating inventions.
With ease, it protects us against billions of bacteria, viruses, and other
parasites. Most of us never reflect upon the fact that while we hang out with
our friends, watch TV, inside our bodies, our immune system is constantly on
the alert, attacking at the first sign of an invasion by harmful organisms. The
immune system is very complex. It's made up of several types of cells and
proteins that have different jobs to do in fighting foreign invaders. The immune
system is composed of many interdependent cell types that collectively protect
the body from bacterial, parasitic, fungal, viral infections and from the
growth of tumor cells. Many of these cell types have specialized functions. The
cells of the immune system can engulf bacteria, kill parasites or tumor cells,
or kill viral-infected cells.
Although rather long and complex, our presentation is just a
glimpse of the immune system and the intricate ways in which its various parts
interact. Immunity is a fascinating subject that still conceals many secrets. When
the immune system is fully understood, it will most likely hold the key to
ridding humankind of many of its most feared diseases.






.jpg)
No comments:
Post a Comment